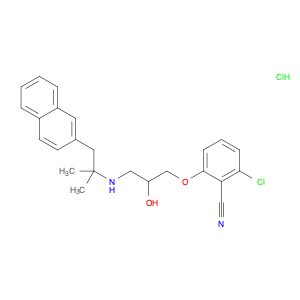
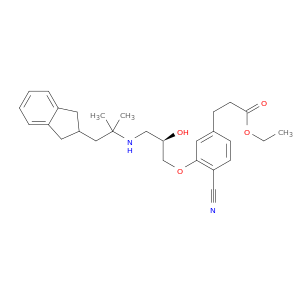
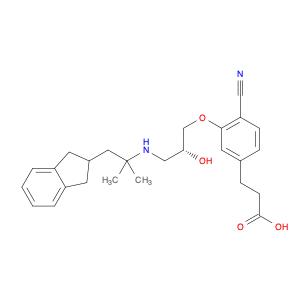
-
Signaling Pathways
- Anti-infection
- Antibody-drug Conjugate/ADC Related
- Cell Cycle/DNA Damage
- Cytoskeleton
- Epigenetics
- GPCR/G Protein
- Immunology/Inflammation
- JAK/STAT Signaling
- MAPK/ERK Pathway
- Membrane Transporter/Ion Channel
- Metabolic Enzyme/Protease
- Neuronal Signaling
- PI3K/Akt/mTOR
- PROTAC
- Protein Tyrosine Kinase/RTK
- Stem Cell/Wnt
- Vitamin D Related/Nuclear Receptor
Anti-infectionAntibody-drug Conjugate/ADC RelatedCell Cycle/DNA DamageCytoskeletonEpigeneticsGPCR/G ProteinImmunology/InflammationJAK/STAT SignalingMAPK/ERK PathwayMembrane Transporter/Ion ChannelMetabolic Enzyme/Protease- 11β-HSD
- 15-PGDH
- 5 alpha Reductase
- Acetolactate Synthase (ALS)
- Acetyl-CoA Carboxylase
- Acyltransferase
- Adiponectin Receptor
- Aldehyde Dehydrogenase (ALDH)
- Aldose Reductase
- Aminoacyl-tRNA Synthetase
- Aminopeptidase
- Amylases
- Angiotensin-converting Enzyme (ACE)
- Arginase
- ATGL
- ATP Citrate Lyase
- Carbonic Anhydrase
- Carboxypeptidase
- Cathepsin
- CETP
Neuronal SignalingPROTACProtein Tyrosine Kinase/RTKStem Cell/WntVitamin D Related/Nuclear Receptor -
Research Areas
- Cancer
- Cardiovascular
- Cell Biology
- Epigenetics
- Metabolism
- Developmental Biology
- Immunology
- Microbiology
- Neuroscience
- Signal Transduction
- Stem Cells
CancerCardiovascularCell BiologyEpigeneticsMetabolismDevelopmental BiologyImmunologyMicrobiologyNeuroscienceSignal Transduction -
Biochemicals
- Amino Acids
- Analytical Standards
- Antibiotics
- Antifungals
- Antioxidants
- Antiparasitics
- Antivirals
- Bile Acids
- Carbohydrates
- Carnitines
- DNA Alkylating Agents
- Drugs of Abuse
- Free Radical Generators
- Homoserine Lactones
- Hydrogen Sulfide Donors
- Ion Channel Modulation
- Isotopically Labeled Standards
- Kinase Inhibitors
- Lab Reagents
- Labeling & Detection
- Lipids
- Metal Chelators
- Microtubule Dynamics
- Natural Products
- Nitric Oxide Donors
- Nucleotides/Nucleosides
- PROTACs
- Peptides
- Pesticides
- Pharmaceutical Impurities
- Phosphoinositides
- Receptor Pharmacology
- Small Molecule Activators
- Small Molecule Inhibitors
- Synthetic Intermediates & Building Blocks
- Toxins
- Transporter & Exchanger Modulators
- Xenobiotic Metabolites
Amino AcidsAnalytical StandardsAntibioticsAntifungalsAntioxidantsAntiparasiticsAntiviralsBile AcidsCarbohydratesCarnitinesDNA Alkylating AgentsDrugs of AbuseFree Radical GeneratorsHomoserine LactonesHydrogen Sulfide DonorsIon Channel ModulationKinase Inhibitors- ATM
- ATR
- Abl Family & Bcr-Abl
- Activin-like Kinases (ALKs)
- Aurora
- CDKs
- CK1
- CK2
- CaMKs
- Carbohydrate Kinases
- Checkpoint Kinases
- DNA-PK
- Dual Specificity Kinases
- EGFR/ErbB/HER Family
- ERK
- FAK Family
- FGFR Family
- GRKs
- GSK3
- HGFR Family
- IGF-1R/InsR Family
- IKKs
- IRAKs
- JAK Family
- JNK
- LIMKs
- LRRKs
- LTK Family
- MAP3Ks
- MEK
- Negative Controls
- Neurotrophin Receptor/Trk Family
- Other Lipid Kinases
- Other Non-Receptor Tyrosine Kinases
- Other Receptor Tyrosine Kinases
- Other Serine/Threonine Kinases
- PAKs
- PDGFR Family
- PDPK1/PDK1
- PI3K
- PIMs
- PKA
- PKB/Akt
- PKC
- PKD & PKG
- Plks
- RAF
- RET
- RIPKs
- ROCK
- S6K
- SRC Family
- Syk Family
- TAM Family
- Tec Family
- Tie Family
- VEGFR Family
- mTOR
- p38 MAPK
Lab ReagentsLabeling & DetectionLipids- Docosanoids
- Fatty Acid Conjugates
- Fatty Acids
- Fatty Alcohols/Aldehydes
- Fatty Amides
- Fatty Esters/Ethers
- Glycerolipids
- Glycerophospholipids
- Hydrocarbons
- Hydroxy/Hydroperoxy/ Epoxy/Oxo Eicosanoids
- Isoprostanes
- Leukotrienes
- Lipoxins/Resolvin Es
- Octadecanoids
- Polyketides
- Prenol Lipids
- Prostaglandins
- Sphingolipids
- Sterol Lipids
- Thromboxanes
Metal ChelatorsMicrotubule DynamicsNatural ProductsNitric Oxide DonorsNucleotides/NucleosidesPROTACsPeptidesPesticidesPharmaceutical ImpuritiesPhosphoinositidesReceptor PharmacologySmall Molecule ActivatorsSmall Molecule Inhibitors- ATPases
- Acetylcholine Turnover
- Acetyltransferases
- Acyltransferases
- Amino Acid Turnover
- Angiotensin-converting Enzyme
- Blood Coagulation Factors
- Carbonic Anhydrase
- Caspases
- Chromatin Reader Interactions
- Cyclooxygenases
- Cytochrome P450
- DNA Repair Enzymes
- Deacetylases
- Deiminases
- Demethylases
- Fatty Acid Metabolism
- GTPases
- Glycerolipid Lipases
- Heat Shock Proteins
- Hydrogen Sulfide Synthesis
- Kinases
- Lipoxygenases
- MMPs
- Methyltransferases
- Negative Controls
- Nitric Oxide Synthases
- Nucleic Acid Turnover/Signaling
- Oxidative Phosphorylation
- Peptidases & Proteases
- Phosphatases
- Phosphodiesterases
- Phospholipases
- Prolyl Hydroxylation Enzymes
- Prostaglandin Synthases
- Reverse Transcriptases
- Sphingolipid Turnover
- Sterol Biosynthesis
- TNF-α/NF-κB Signaling
- Topoisomerases
- Ubiquitin Ligase System
Synthetic Intermediates & Building BlocksToxinsTransporter & Exchanger ModulatorsXenobiotic Metabolites - Ordering & Shipping
- My Account
 Login
Login